Can L Glucose Form a Closed Structure
Unlike D-glucose the oxygen and hydrogen group of atoms in L-glucose points to the left in the Fisher modelOne difference between D-glucose and L-glucose is O the open-chain form of L-glucose does not exist O it is not possible to make L-glucose O L-glucose cannot form a closed structure O only D-glucose can act as a substrate in metabolic reactions O L-glucose has a 5. The atoms in this cyclic molecule then arrange themselves in space to minimize the amount of strain on each of the covalent bonds.
Difference Between D And L Glucose Definition Structure Properties
L-Glucose does not occur naturally in living organisms but can be synthesized in the laboratory.

. Fructose can only be metabolized by the liver that is why it should not be taken in a large quantity. However L-Glucose is a low-calorie sweetener that is a good suggestion for diabetes patients. 3 Simple Steps the Ring Structure of Glucose Molecule.
Draw extended arms for all the carbon atoms excluding the first one. The α form rotates polarized light clockwise to the right by. The first carbon atom C1 which is an aldehyde group -CHO creates a hemiacetal with the fifth carbon atom C5 to make a 6-membered-ring termed a pyranose.
Glucose can be metabolized by every cell in living organisms. Now we know that over 99 percent of the time glucose occurs in the closed-ring form. In total there are 24 atoms that form the molecule.
Chemistry questions and answers. A primary source of energy for living organisms. Only the carbon outside the ring number 6 has 2 single hydrogens and an OH group.
L-Glucose is indistinguishable in taste from d-glucose but cannot. Glucose are hexoses 6 carbon atoms sugars. For drawing purposes the ring structure of the glucose molecule is broken down into three simple steps as shown below.
Aldehydo-L-glucose is the L-enantiomer of aldehydo-glucose. Glucose c6h12o6 can exist as both an open-chain form and a closed-ring form. Only D-glucose can act as a substrate in metabolic reactions.
It is a L-glucose and an aldehydo-glucose. One difference between D-glucose and L-glucose is athe open chain form of L-glucose does not exist bL-glucose cannot form a closed structure cit is not possible to make L-glucose dL-glucose has a 5 membered ring and a D-glucose has a six membered ring e. Linear glucose has four chiral carbons pointed to by the red arrows.
Its chemical formula is C 6 H 12 O 6. L-Glucose is an organic compound with formula C 6 H 12 O 6 or OCHCHOH 5 H specifically one of the aldohexose monosaccharidesAs the l-isomer of glucose it is the enantiomer of the more common d-glucose. The ring itself is 6-sided but only 5 of its corners are made up by carbon atoms.
Since linear and cyclic forms can inter-convert however no glucose molecule is ever fixed in the six-membered ring form. Follow the steps given below to draw an acyclic form of glucose. The rest is one of two cyclic forms of glucose formed when the hydroxyl group on carbon 5 C 5 bonds to the aldehyde carbon 1 C 1 as shown below.
It can go back and forth. D and L isomers are stereoisomers that have the same chemical structure but are non-superimposable mirror images of each other. D-glucose and L-glucose are composed.
Furthermore D-glucose can exist in both linear form and cyclic form but L-glucose exists in an equilibrium mixture of α-L-glucopyranose and β-L-glucopyranose. The glucose molecule in this form is known as α-D. Glucose is a sugar molecule that is found as either D-Glucose or L-Glucose in natureThe main difference.
Main Difference D vs L Glucose. One difference between D-glucose and L-glucose is O the open-chain form of L-glucose does not exist O it is not possible to make L-glucose O L-glucose cannot form a closed structure O only D-glucose can act as a substrate in metabolic reactions O L-glucose has a 5-membered ring and D-glucose has a 6-membered ring A Click Submit to complete this assessment. This makes them susceptible to nucleophilic attack in which an atom with unshared electron pairs is.
Difference between L-Glucose and D-Glucose L-Glucose Chemistry. Glucose molecules form rings. Isomerism is divided into two broad categories as structural isomerism and stereoisomerism.
Draw 6 carbon atoms. D-glucose can exist in the straight-chain form above and as four different cyclic structures. It is used therapeutically in fluid and.
The glucose molecule can exist in an open-chain acyclic and ring cyclic formin equilibrium the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. It is an enantiomer of an aldehydo-D-glucose. The six-membered ring is much more common and in solution the vast majority of glucose molecules are found to have six-membered rings.
Glucose C 6 H 12 O 6. Now draw hydrogen to carbon bond such that four are on one side and one on the other side. The linear form of glucose shown above makes up less than 3 of the glucose molecules in a water solution.
The pyranose part of the name tells us that it is a six-membered ring. In solution it consists of an equilibrium mixture of α-D-glucopyranose and β-D-glucopyranose. Steps to Draw Open Chain Structure of a Glucose Molecule.
In solutions the open-chain form of glucose either D- or L- exists in equilibrium with several cyclic isomers each containing a ring of carbons closed by one oxygen atom. Glucose is a carbohydrate and it is one of the smallest units of sugar. It is naturally occurring and is found in fruits and other parts of plants in its free state.
Therefore it is the most preferred form of energy by the body. L-glucose is the mirror image of D-glucose. Summary D vs L Glucose.
Glucose can form either five-membered or six-membered rings. One difference between D-glucose and L-glucose is L-glucose cannot form a closed structure only D-glucose can act as a substrate in metabolic reactions L-glucose has a 5-membered ring and D-glucose has a 6- membered ring the open-chain form of L-glucose does not exist it is not possible to make L-glucose. The glucose molecule can form into other configurations but this structure - a ring or chair form - is the most stable and therefore most common in biological systems.
Answer 1 of 2. D-glucose is the main energy source in most of the living organisms. Because oxygen is much more electronegative than carbon carbonyl groups are strongly polarized with the carbon being partially positive electron deficient.
Before 1900 glucose was only thought to occur as an open chain. In aqueous solution however more than 99 of glucose molecules exist as pyranose forms. Each molecule of glucose sugar is only 1 unit consisting of 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in the form of a ring or a straight chain.

Cyclic Structure Of Glucose Definition Examples Diagrams

D And L Glucose Are Enantiomers Chemistry Science Chemistry Organic Chemistry
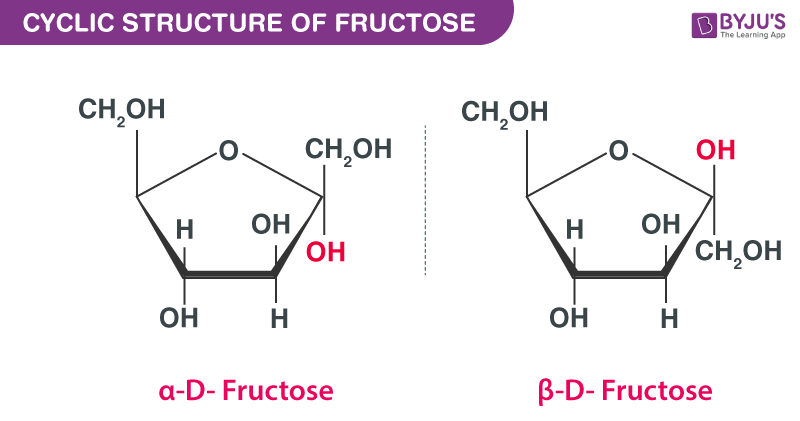
Structure Of Glucose And Fructose Properties Isomers Steps To Draw The Structure Of Glucose And Fructose

Solved Compare The Structures Of D Glucose L Glucose Chegg Com
Difference Between D And L Glucose Definition Structure Properties

Organic Chemistry In The Haworth Projections Of D And L Glucose Is The Stereochemistry At Every Carbon Except The Anomeric Carbon Just Inverted Chemistry Stack Exchange
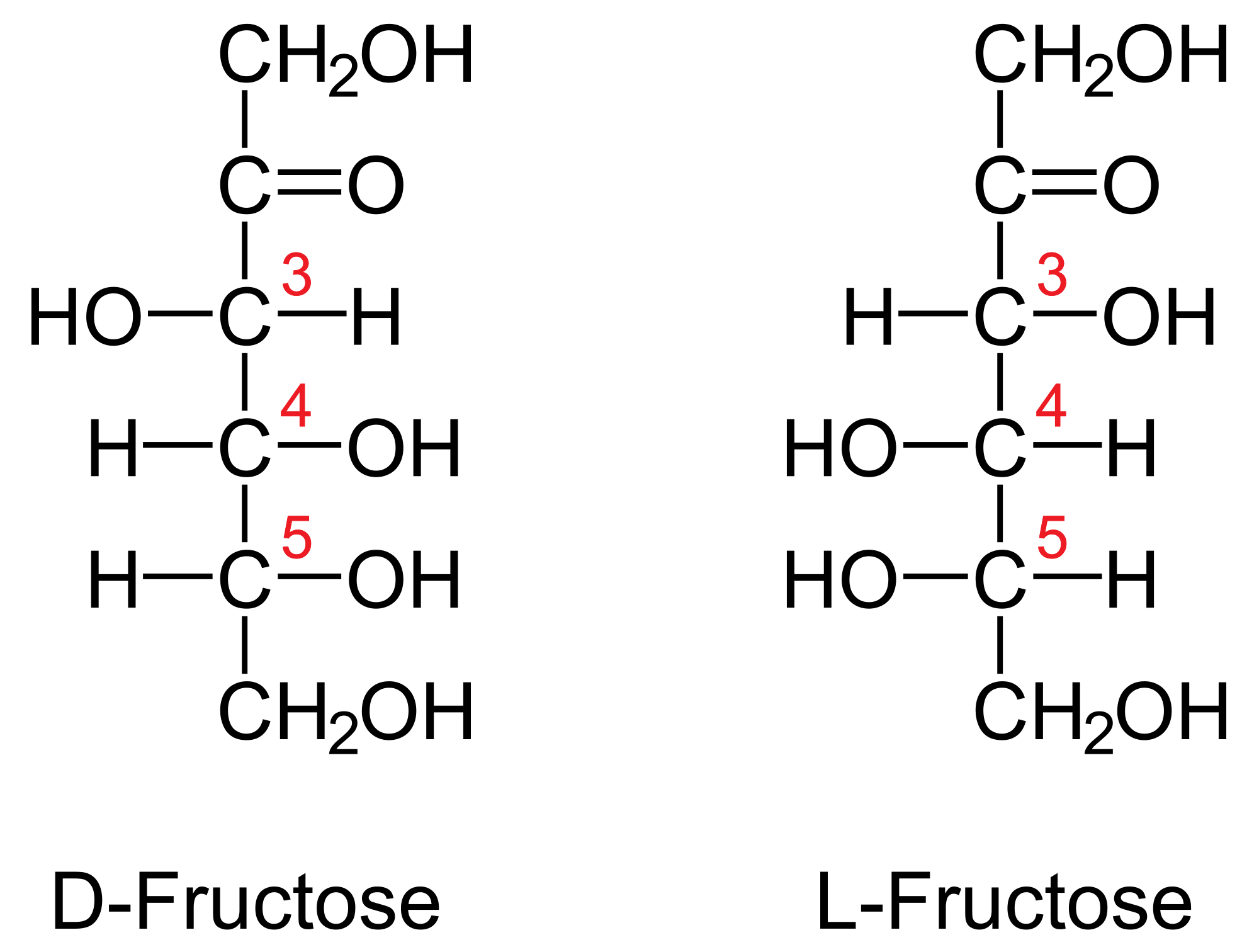
If D And L Glucose Are Enantiomers Are D And L Fructose Not Enantiomers Socratic
Difference Between D And L Glucose Definition Structure Properties
Difference Between D And L Glucose Definition Structure Properties

Difference Between D And L Glucose Compare The Difference Between Similar Terms

Difference Between D And L Glucose Compare The Difference Between Similar Terms
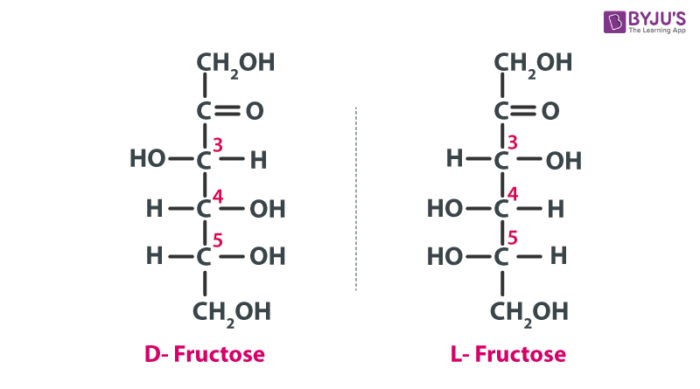
Structure Of Glucose And Fructose Properties Isomers Steps To Draw The Structure Of Glucose And Fructose

Glucose Cyclic Structure Chemistry Lecture Chemistry Textbook Teaching Chemistry
What Is Difference Between D Glucose And L Glucose Quora
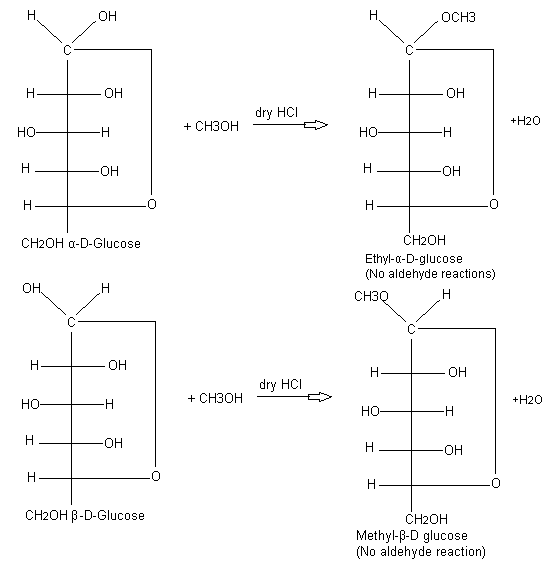
Glucose Structure Diagrams Examples Physical Properties

Organic Chemistry In The Haworth Projections Of D And L Glucose Is The Stereochemistry At Every Carbon Except The Anomeric Carbon Just Inverted Chemistry Stack Exchange


Comments
Post a Comment